atalysmedical@gmail.com
Accelerating Cycle Times for Medical Device Manufacturing
Efficient thermal management is crucial in the production of medical devices and drug delivery systems, where up to 80% of the injection molding cycle is consumed by cooling. This Atalys presentation at SPE/MPD's Minitec at MD&M West in Anaheim California introduces a robust methodology for designing cooling systems using steady-state response thermal models, aimed at significantly reducing cooling times and boosting manufacturing efficiency. By precisely quantifying cooling challenges and optimizing key design parameters, this approach provides a streamlined modeling strategy for rapid and effective analysis.
Elevating the Future of Medical Device Manufacturing
With the acquisition of Schnipke Precision Molding, Atalys deepens its core competencies—in extreme precision tooling, optimized plastics processing and molding, and automated components assembly—further extending its leadership in minimally invasive and robotic assisted surgery, surgical access, instruments, energy, and drug delivery markets.
Atalys Completes the Integration of Romold
With the integration of Romold-- highly regarded as a top quality mold maker with an experienced team and workforce-- Atalys’ Advanced Engineering Center in Rochester, NY, which is ISO 9001:2015 certified, now has an expanded capacity to build in excess of 250 extreme precision, high cavitation, and complex-geometry molds per year.
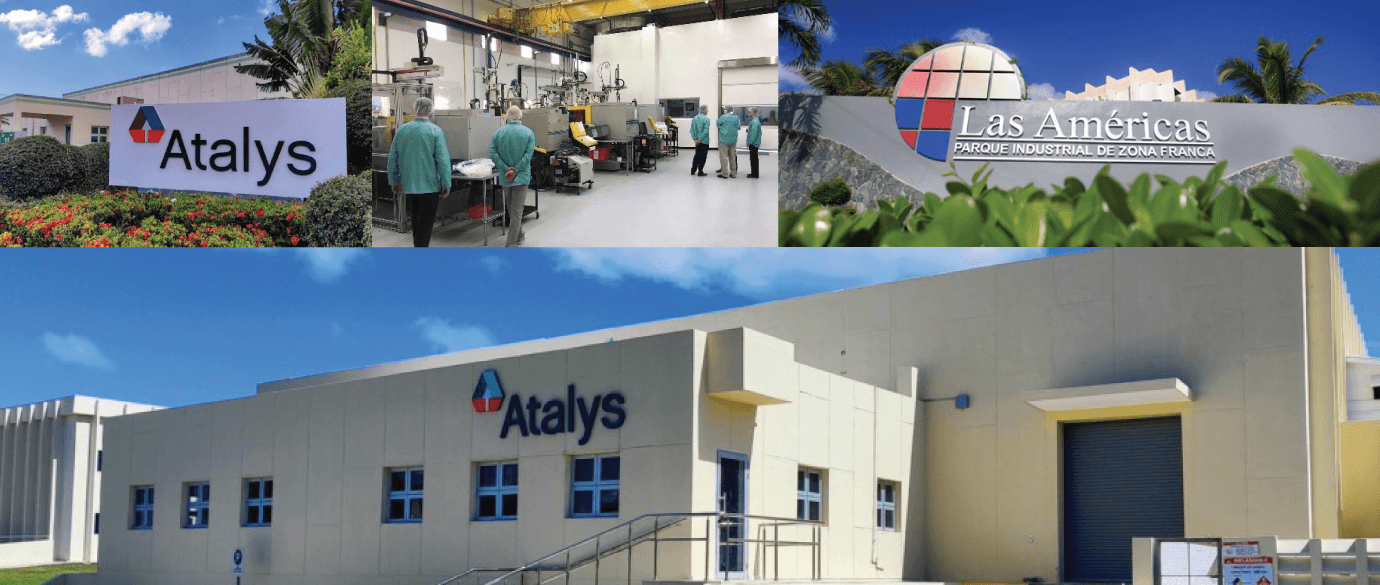
Atalys Expands Manufacturing Facility in the Dominican Republic
The new adjacent building adds 32,000 square feet of manufacturing capacity and value-added operations to the existing site. Atalys Dominican Republic is located within Las Americas Free Zone Park, a high-tech tax-free zone that is located less than 15 miles from a full-service port with excellent transportation and logistics infrastructure.