Plastic Injection Molding
Accelerating Cycle Times for Medical Device Manufacturing
Efficient thermal management is crucial in the production of medical devices and drug delivery systems, where up to 80% of the injection molding cycle is consumed by cooling. This Atalys presentation at SPE/MPD's Minitec at MD&M West in Anaheim California introduces a robust methodology for designing cooling systems using steady-state response thermal models, aimed at significantly reducing cooling times and boosting manufacturing efficiency. By precisely quantifying cooling challenges and optimizing key design parameters, this approach provides a streamlined modeling strategy for rapid and effective analysis.
Optimizing Mold Cooling for Medical Plastics: Insights from a Leading Expert
In this exclusive interview, Dr. Crispino explores the science behind cure time optimization, advanced thermal management, and the latest cooling methods to ensure precision and efficiency in medical device manufacturing. Learn about innovative modeling techniques, materials analysis, and actionable insights that can transform your mold design process. Ideal for professionals in engineering, R&D, manufacturing, and procurement, this interview provides insights into how you can improve cycle times and product quality.
Optimizing Supply Chain Resilience in Robotic-Assisted Surgery Device Manufacturing
Whether you are an emerging robotic-assisted surgery company bringing new technologies to market, or looking to accelerate production or transitioning from prototype to full-scale manufacturing, Atalys is your strategic partner in navigating complex global supply chains and ensuring your precision components are not only delivered on time, but more importantly manufactured according to your specifications.
The Power of Plastic Injection Molding: Why It’s the Ideal Choice for Medical Device Companies
The medical device industry is constantly evolving, driven by the demand for innovative solutions to improve patient outcomes and streamline healthcare processes. In this highly regulated and competitive environment, manufacturers must rely on advanced manufacturing techniques to produce high-quality, reliable, and cost-effective products.
Major Design Rules for Plastic Injection Molding Medical Device Parts
Designing plastic parts for medical devices can be a complex and challenging process, especially when it comes to achieving tight tolerances, high precision, and optimal functionality. This guide explores the major design rules and provides tips and insights on how to optimize your part’s design for maximum efficiency and quality.
Elevating the Future of Medical Device Manufacturing
With the acquisition of Schnipke Precision Molding, Atalys deepens its core competencies—in extreme precision tooling, optimized plastics processing and molding, and automated components assembly—further extending its leadership in minimally invasive and robotic assisted surgery, surgical access, instruments, energy, and drug delivery markets.
Atalys Completes the Integration of Romold
With the integration of Romold-- highly regarded as a top quality mold maker with an experienced team and workforce-- Atalys’ Advanced Engineering Center in Rochester, NY, which is ISO 9001:2015 certified, now has an expanded capacity to build in excess of 250 extreme precision, high cavitation, and complex-geometry molds per year.
Molding No Draft Tubes
Medical device parts with cylindrical shapes are traditionally designed to have tapers in both internal and external walls which facilitate easy ejection of the molded part from the tool without any issues. There are times, however, when a part’s required tolerances and/or function will not allow for it. This was the case with a customer who wanted a tubular component molded without drafts under strict tolerances. Could the part be molded without any drafts? Find out in this case study.
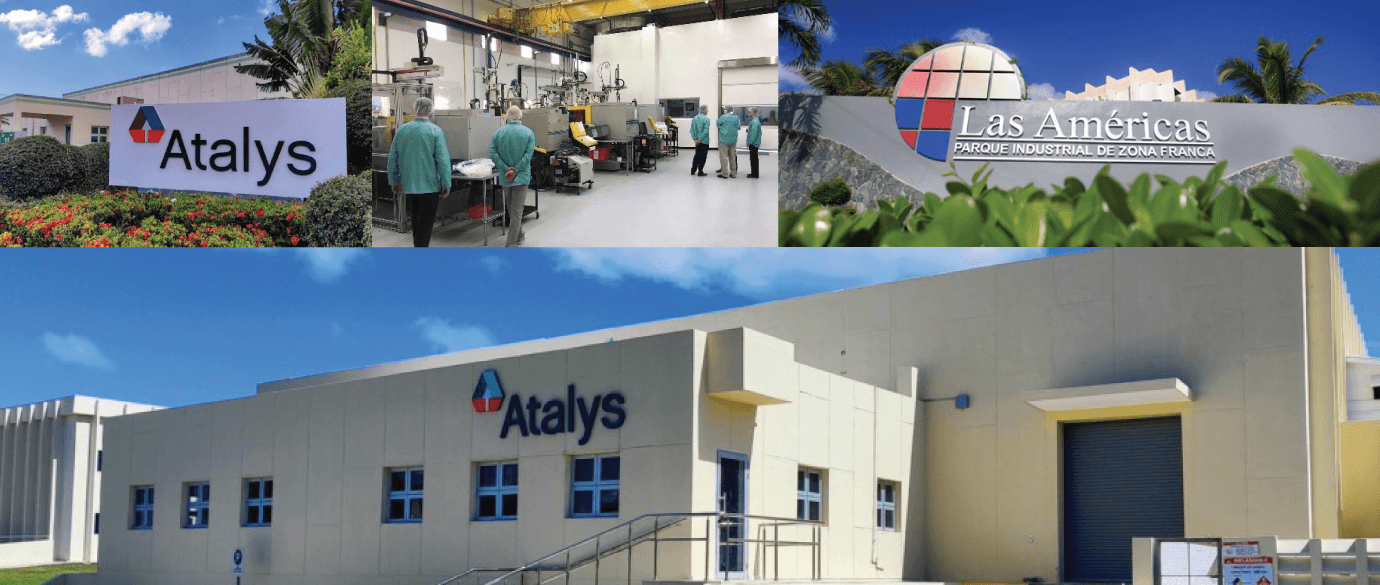
Atalys Expands Manufacturing Facility in the Dominican Republic
The new adjacent building adds 32,000 square feet of manufacturing capacity and value-added operations to the existing site. Atalys Dominican Republic is located within Las Americas Free Zone Park, a high-tech tax-free zone that is located less than 15 miles from a full-service port with excellent transportation and logistics infrastructure.
Is Two-Shot Molding Right for Your Part?
One process that has gained prominence in recent years is two-shot plastic injection molding. It allows for the creation of complex parts in a single manufacturing step, in the process reducing assembly time and costs, as well as improving the aesthetics and functionality of the final product, and in the end improving patient outcomes. But is it right for your part?