

WHERE TO FIND US
Enhancing Injection Mold Cooling Efficiency with Steady State Thermal Models and Composite Core Technology: Accelerating Cycle Times for Medical Device Manufacturing
Efficient thermal management is crucial in the production of medical devices and drug delivery systems, where up to 80% of the injection molding cycle is consumed by cooling. This presentation introduces a robust methodology for designing cooling systems using steady-state response thermal models, aimed at significantly reducing cooling times and boosting manufacturing efficiency. By precisely quantifying cooling challenges and optimizing key design parameters, this approach provides a streamlined modeling strategy for rapid and effective analysis.
SPEAKER: DR. DAVID CRISPINO, VP OF R&D
Tuesday, February 4, 2025
4:00 PM – 4:30 PM (PST)
Anaheim Convention Center Level 2
Room 201 C&D
801 West Katella Avenue
Aneheim, CA 92802
WHY ATTEND
In the fast-evolving world of medical device manufacturing, success hinges on precision, efficiency, and innovation. For medical plastics engineers and manufacturing professionals tasked with developing high-performance components for medical devices, optimizing the injection molding process is critical—not just to improve cycle times, but to deliver exceptional product quality and reliability. Cure time accounts for the majority of cycle time, representing a major bottleneck in the injection molding cycle and production efficiency. This presentation offers a deep dive into a novel approach and advanced methodology designed to address this challenge head-on.
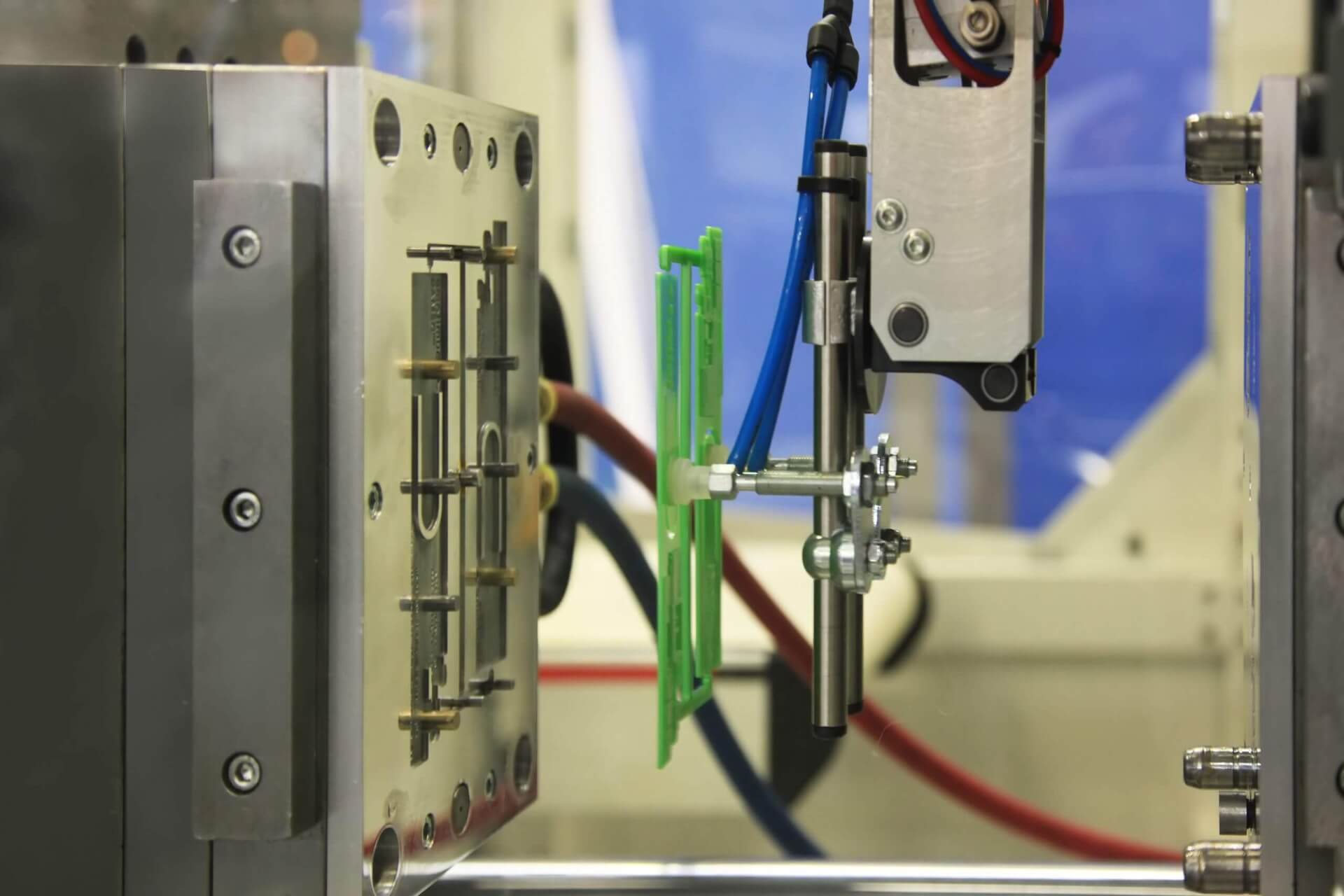

WHO SHOULD ATTEND
This session is ideal for professionals in engineering, R&D, manufacturing, and procurement who are eager to leverage cutting-edge solutions in building and operating injection molds to stay competitive in a demanding market. These include tool designers, tool engineers, plastic engineers, process engineers, and purchasing managers. Whether you are seeking to refine your current processes or exploring new innovations in medical plastics, this presentation will equip you with the tools and knowledge to elevate your operations and deliver greater value to your organization and customers.
WHAT YOU’LL LEARN
Session attendees will gain the following:
- Proven Strategies for Thermal Management: Discover how steady-state thermal models can quantify cooling challenges with accuracy and drive informed decisions for system design optimization, as well as gain exposure to composite core technologies that are revolutionizing cooling system efficiency and delivering unmatched value for medical device OEMs.
- Real-World Case Studies: Explore practical examples of how these techniques have been used to design cooling systems that delivered measurable improvements in cycle times, quality, and overall productivity for high-precision medical device components.
- Actionable Insights: Learn how to accelerate mold design and validation timelines, streamline debugging, and expand process windows to achieve not only enhanced robustness and consistency but also support scalable manufacturing processes.

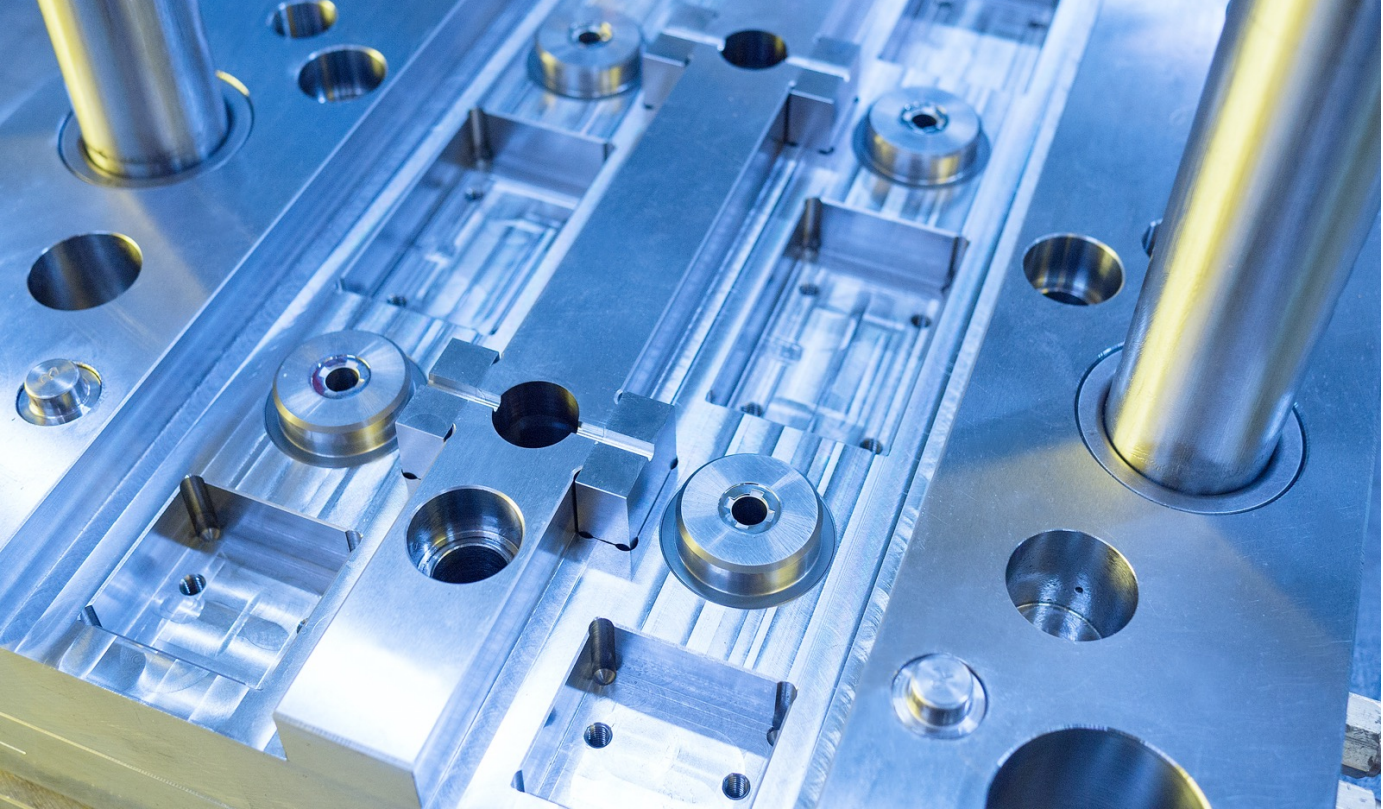
HOW THIS PRESENTATION IMPACTS THE INDUSTRY
This presentation addresses a critical aspect of medical device manufacturing that influences the entire industry: the efficiency and precision of injection molding processes. Cure time optimization is not just a manufacturing concern—it is a cornerstone of competitive advantage, sustainability, and innovation in the medical device sector. By addressing the most time-consuming and technically demanding aspects of injection molding, this session empowers professionals to drive real-world improvements that ripple through the entire supply chain—from manufacturers to patients. These innovations not only help individual OEMs meet current demands but also position the industry to tackle future challenges with confidence and resilience.
OUR EXPERT

DR. DAVID CRISPINO
Dr. Crispino is a seasoned technical leader with over forty years of experience in the injection molding industry, with a special focus on medical plastics. As Vice President of R&D at Atalys, where he leads the Advanced Engineering Group, he focuses on providing in-depth analysis and modeling of structural and thermal challenges associated with the design and manufacturing of extreme precision plastic components for medical devices. He holds a PhD in Mechanical Engineering and is recognized for his deep technical insight and leadership in driving innovation within the medical plastics manufacturing industry.


